We at Beyond Next Ventures have made a new investment in the medical device startup GramEye from our deep-tech focused Fund III. The startup, led by Dr. Yu Hiraoka and Dr. Tatsuya Yamada, aims to address the problem of antimicrobial resistance by transforming the method of how infectious diseases are diagnosed through the power of AI and robotics.
GramEye is currently commercializing an automated Gram staining and analysis system. Gram staining is a test conducted to diagnose infections. Currently, antimicrobial resistance is an enormous problem in our society, but we firmly believe that GramEye can make a significant contribution toward its solution.
In this article, we explain the background behind our investment in GramEye.
Contents
The Global Public Health Challenge of Antimicrobial Resistance (AMR)
When bacterial infections such as pneumonia or urinary tract infections are suspected, patients take medicine known as antimicrobial agents.
When administered appropriately, antimicrobials can eradicate the causative bacteria and cure infections. However, the inappropriate use of antimicrobials fails to eliminate bacteria completely, and drug-resistant bacteria survive and in some cases they also proliferate. These are known as antimicrobial-resistance or in simple words drug-resistance. As antimicrobial-resistance increases, the prevention and treatment of infections become more difficult, raising the risk of severe illness and death.
Antimicrobial resistance affects large numbers of people worldwide, both clinically and economically. It has been projected that by 2050, deaths caused by antimicrobial-resistant infections could reach 10 million worldwide [2], exceeding the current number of deaths from malignant tumors (cancer). The global GDP has also been estimated to be reduced by as much as 3.8% [3] resultantly.
Since the World Health Assembly adopted the WHO Global Action Plan on Antimicrobial Resistance in 2016, clear goals and action plans have been established to address this issue. In 2023, Japan’s Ministry of Health, Labour and Welfare announced the promotion of the appropriate use of antimicrobial drugs, along with the introduction of market incentives for antimicrobial drug development.
Japan introduced a “pull incentive” system in 2023, which provides post hoc rewards to pharmaceutical companies that have successfully developed new antimicrobials. Furthermore, in 2024, new reimbursement schemes were established for medical institutions that monitor antimicrobial use and prescribe them appropriately.
Nevertheless, the targets for appropriate use outlined in these action plans have yet to be achieved. Antimicrobial resistance continues to spread, with reports indicating that approximately one-sixth of laboratory-confirmed bacterial infections worldwide in 2023 were caused by antimicrobial-resistant organisms [5].
The Importance of Appropriate Prescription of antimicrobial and Gram Staining
While antimicrobials have saved countless lives, they have also given rise to the new challenge of antimicrobial resistance. Until the 20th century, resistance was addressed primarily through the development of new antimicrobials with novel mechanisms of action. However, in recent years this field has reached a mature stage, and the number of approvals for new antimicrobials – based on novel compounds – has been steadily declining.
A timeline of antimicrobial drug development and antimicrobial resistance[6]
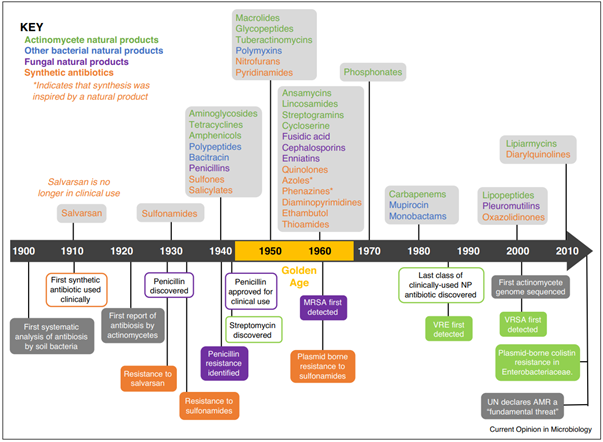
It may be right to say that this is no longer an era like the 1980s, when many new antimicrobial agents were developed. As a result, appropriate prescription of antimicrobials—aimed at preventing the emergence of resistant bacteria in the first place—has become increasingly important. But what exactly is the appropriate prescription of antimicrobial?
While inappropriate administration may be the result of missed doses or premature discontinuation by patients, here we focus on the clinical perspective.
When treating infections, physicians consider the mode of onset, the infected organ, disease severity, patient background, and various test results to determine the likely causative pathogen. Based on this assessment, they prescribe antimicrobials deemed appropriate for the suspected organism.
From the antimicrobials that are considered empirically effective for each clinical situation, we determine which widely usable antimicrobials to prescribe by weighing the risks and benefits. This type of experience-based selection of antimicrobial therapy is referred to as “empiric therapy.”
Empiric therapy is based on a large body of evidence and is indispensable, particularly in severe cases where there is no time to wait for diagnostic test results. On the other hand, the broader the spectrum of the prescribed antimicrobial drug is, the greater the risk of overtreatment, adverse effects, and the emergence of antimicrobial resistance.
This is where diagnostic tests such as “Gram staining” and “bacterial cultivation studies” become critical.
Gram Satining; Gram staining, as its name suggests, is a test that stains cells. Although it is a classical technique and can be performed in as little as 5–10 minutes per sample when expedited, it provides remarkably powerful information.
Different types of bacteria have different shapes (cocci or bacilli), and differences in their cell wall structure determine the color they take on staining (Gram-positive or Gram-negative). Some bacteria also exhibit distinctive clustering patterns or boundary characteristics. For example, some form chains, while others cluster in grape-like formations. In some cases, Gram staining can even provide information that nearly identifies the bacterial species.
Bacterial Culture Testing; By contrast, bacterial culture testing involves collecting specimens from the site of infection—such as sputum, blood, urine, or pus—and cultivating the bacteria present in the sample. Once the bacteria have grown, the species is identified. This is a definitive test that can determine the exact bacterial species, and it also assesses the antimicrobial susceptibility of the bacteria (i.e., determining which antimicrobials are effective). In this sense, it serves as a means of “confirming the correct answer” in antimicrobial selection.
However, because the process requires time for bacterial growth, it typically takes about 2–5 days to obtain results. When the results of bacterial culture testing become available, antimicrobial therapy is revised as needed. Switching to a narrower-spectrum antimicrobial is referred to as de-escalation, and it is an important practice for preventing adverse effects and the emergence of antimicrobial-resistantance.
In other words, appropriate antimicrobial prescribing is highly complex: it requires evidence-based empiric antimicrobial selection, prompt initial treatment decisions informed by morphological and staining characteristics obtained through rapid Gram staining, and subsequent reassessment of therapy based on bacterial cultivation results.
Despite its critical role, Gram staining faces significant challenges.
Challenges of Gram Staining
Hospitals have facilities such as clinical laboratories or central laboratories, where specimens from throughout the hospital are continuously delivered around the clock. Laboratory technologists are required to perform Gram staining while also handling a wide range of other tests, including blood tests, urinalysis, and examinations needed for blood transfusions.
Of course, depending on the size of the hospital, some facilities may have a certain degree of task specialization or a separate microbiology laboratory. However, at least in Japan, it is rare to find someone who is able to focus exclusively on Gram staining throughout the day.
Conventional Gram staining requires a series of simple but labor-intensive steps: applying the specimen to a slide, fixation, two-step staining with different dyes, with rinsing after each step, drying, and finally microscopic observation. Because all steps are performed manually, variations can occur in staining results depending on immersion and decolorization times. In addition, interpretation of stained slides under the microscope may vary in accuracy depending on the observer.
Although the procedure itself can be completed in about 5–10 minutes if conducted by someone experienced, it requires the technologist to remain at the washing station throughout that time, preventing them from performing other tests. Therefore, although Gram staining itself is a simple procedure, there is a gap between the ease of performing the test and the time required to report the results; in practice, it often takes from half a day to two days for the results to be out.
Moreover, many hospitals do not operate Gram staining services 24 hours a day, 365 days a year, and the test is often unavailable at night or on holidays.
In both outpatient and inpatient settings, physicians usually cannot wait even half a day to initiate treatment. As a result, they are forced to begin therapy based solely on empiric considerations. This can lead to inappropriate antimicrobial prescribing, such as the excessive use of broad-spectrum antimicrobials or, in the worst cases, prescribing antimicrobials that are ineffective. These practices in turn promote the emergence of antimicrobial-resistant bacteria and drive up healthcare costs.
This challenge is addressed by Mycrium, a medical device developed by GramEye that leverages the power of AI and robotics.
The Automated Gram Staining and Analysis Device “Mycrium”

Mycrium consists of two main components. First, a specimen collected from a patient is smeared and fixed onto a slide, which is then placed into a Mycrium slot. The slide is automatically drawn into the system, where a staining robot performs Gram staining. Subsequently, an internally mounted microscope and AI system automatically examine the slide, detect and image bacterial cells, and classify and infer bacterial species based on Gram staining results. The results are then delivered to the laboratory technologist. The technologist simply reviews the images and results on a PC, verifies them, and submits the final report to the physician.
By fully automating both the staining and analysis processes that were traditionally performed manually, Mycrium significantly reduces the burden on laboratory technologists. Once the slide is placed in the slot and the system is activated, technologists are free to perform other tasks. Unlike traditional workflows, they no longer need to be fully occupied with Gram staining, enabling faster and more convenient reporting of results.
However, Mycrium offers benefits beyond reducing technologists’ workload.
From a physician’s perspective, if results can be returned within 30 minutes to an hour, this is comparable to the turnaround time for other tests such as blood work. This enables physicians to incorporate Gram staining results into their treatment planning, which in turn contributes to improved clinical outcomes [10] and reduces unnecessary use of broad-spectrum antimicrobials [11].
Hospital administrators can also expect tangible benefits.
When the practice of appropriate prescription of antimicrobials – based on Gram staining– is thoroughly implemented, then reimbursement incentives become available only to such hospitals (that follow right prescription practices.)
Broad-spectrum antimicrobials are generally pricier than narrow-spectrum agents, and right prescription leads to a reduction in drug costs – and thus directly contributes to improving hospital revenue [12] – especially in DPC hospitals where inpatient costs remain the same regardless of the medications administered.
Furthermore, automation may help reduce overtime for laboratory technologists, enabling them to use the saved time for other tasks.
Mycrium, therefore, provides a solution that benefits all stakeholders involved in infectious disease treatment – patients, physicians, laboratory technologists, and hospital administrators alike. As a fully integrated automated solution for Gram staining, it holds a significant competitive advantage even on a global scale, and as the Japanese government and international organizations move to address the issue of antimicrobial resistance, GramEye has the potential to become a central player in these efforts.
The World GramEye Aims to Create
GramEye began as a student project by CEO Dr. Hiraoka and COO Dr. Yamada, who were then medical students at Osaka University at the time.
After entering medical school, the two sought to make a broader societal impact beyond daily clinical practice. Dr. Hiraoka studied accounting and programming, while Dr. Yamada actively engaged in volunteer activities, including NPO work in ASEAN countries and research on antimicrobial resistance. While touring a hospital laboratory, Dr. Yamada became aware of the challenges associated with Gram staining and proposed to Dr. Hiraoka that these issues could be solved using AI and robotics. The development work began in 2019.
The duo continued development alongside their studies and residency training, and founded the company in 2020. They were soon joined by professionals from major microbiological diagnostic equipment manufacturers and pharmaceutical companies specializing in microbiology.
With this strong team, the business advanced rapidly. In 2025, Mycrium was successfully launched as a medical device. Within less than a year since its release, it’s being introduced at leading large hospitals and testing labs across Japan, with sales ramping up faster than expected.
The primary reason for our investment in GramEye is the founders’ sincere commitment to balancing the resolution of a major societal challenge with economic viability. Antimicrobial resistance, like climate change, is a negative-externality–driven societal issue, and relying solely on clinical correctness or medical ethics is insufficient to achieve widespread adoption.
GramEye engages daily in research and development as well as policy advocacy.
GramEye’s goal is to realize a world in which antimicrobials are prescribed appropriately and the problem of antimicrobial resistance is resolved. This is an ambitious vision, but we firmly believe that the strong team led by Dr. Hiraoka and Dr. Yamada can achieve it and become a flagship company in the fight against antimicrobial resistance.
We at Beyond Next Ventures will fully support them on this journey.
-
List of Sources:
- [1] AMR臨床リファレンスセンター「インフォグラフィックで知る!薬剤耐性(AMR)」
- [2] O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance; 2016.
- [3] World Bank. 2017. “Drug-Resistant Infections: A Threat to Our Economic Future.” Washington, DC: World Bank. License: Creative Commons Attribution CC BY 3.0 IGO.
- [4] 経済財政運営と改革の基本方針2025について
- [5] Global antibiotic resistance surveillance report 2025: WHO Global Antimicrobial Resistance and Use Surveillance System (GLASS). Geneva: World Health Organization; 2025.
- [6] Hutchings, et al., Antibiotics: past, present and future. Current Opinion in Microbiology. 2019;51:72-80
- [7] 厚生労働省「抗微生物薬の市場インセンティブに関する検討会について」に関する資料
- [8] The AMR Action Fund プレスリリース
- [9] 神奈川県衛生研究所 Webサイトより抜粋
- [10] Fukuyama, et al. Validation of sputum Gram stain for treatment of community-acquired pneumonia and healthcare-associated pneumonia: a prospective observational study. BMC Infect Dis. 2014;14:534.
- [11] Yoshimura et al. Effect of Gram Stain–Guided Initial Antibiotic Therapy on Clinical Response in Patients With Ventilator-Associated Pneumonia: The GRACE-VAP Randomized Clinical Trial. JAMA Netw Open. 2022;5(4):e226136.
- [12] Taniguchi, et al. Gram-stain-based antimicrobial selection reduces cost and overuse compared with Japanese guidelines. BMC Infect Dis. 2015;15:458.